
One of the ingredience in nail polish is ALCOHOL.
Anne Marie Helmenstine
Multiple Choice Questions
Q1. Which of the following hydrocarbon family has the highest boiling point?
A. Alkane
B. Halo-alkane
C. Ether
D. Alcohol
Q2. Lower alcohols like methanol burn with a blue flame to form carbon dioxide and water in the presence of atmospheric oxygen. Such a type of reaction is called
A. Combustion
B. Dehydration
C. Esterification
D. Oxidation
Q3. Alcohols are viscous liquids and their viscosity depends on the strength of the inter-molecular forces. The alcohol with the highest viscosity is:
A. Methanol
B. Ethanol
C. Heptanol
D. Octanol
Q4. The molecular formula of butanol is
A. C4H8OH
B. C4H9OH
C. C4H10OH
D. C4H7OH
Q5. The reaction of carboxylic acids with alcohols in the presence of. H2SO4 as a catalyst is
A. Combustion
B. Esterification
C. Oxidation
D. Neutralization
Q6. Which of the following sequence is correct for the oxidation of alcohol?
A. Ethanol→ Ethanal→ Ethanoic acid
B. Ethanol→ Ethanoic acid→ Ethanal
C. Ethanal→ Ethanol→ Ethanoic acid
D. Ethanal→ Ethanoic acid→ Ethanol
Q7. The molecular formula of alcohol having six carbon atoms is
A. C6H10OH
B. C6H11OH
C. C6H12OH
D. C6H13OH
Q8. Police use breath analyzer to detect the level of alcohol in drivers. Which property of alcohol is used in breath analyzers?
A. reducing property
B. oxidising property
C. denaturing property
D. dehydrating property
Q9. The correct structural formula of butanol is
A.

B.
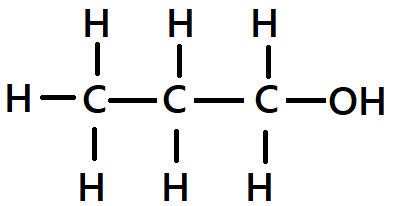
C.

D.

Q10. The IUPAC name for the CH3CH2CH2OH is
A. Methanol
B. Ethanol
C. Propanol
D. Butanol
Questions
Q1. Fill in the blanks:
a) The biofuel mixture of 30% ethanol and petrol is called _______________.
Ans.: gasohol
b) High solubility of ethyl alcohol in water is due to formation of high _______________ hydrogen bonding.
Ans.: intra-molecular
c)

Ans.: 2C₂H₅OH
Q2. Why burning ethanol fuel is considered neutral to the environment?
Ans.: It is because the amount of carbon dioxide released during ethanol production is the same as the amount absorbed by the crops grown as a feedstock.
Q3.Why fractional distillation is used for the rectification of ethanol
Ans.: Because to remove impurities.
Q4. Why ethanol is collected before water during distillation?
Ans.: It is because ethanol has lower boiling than water.
Q5. The chemical equation given below is the examples of:
a)
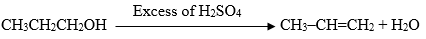
Ans.: DEHYDRATION as there is the removal of a water molecule from the alcohol (propanol)
b)

Ans.: ESTERIFICATION, since there is the formation of ester (CH3COOC2H5)
Q6. Following are the structures of the first three members of a homologous series of alcohols. Based on the structures, answer the following questions:
i) CH3OH ii) CH3CH2OH iii) CH3CH2CH2OH
a) Write the general formula of alcohols.
Ans.: CnH2n+1OH
b) Arrange the three structures in increasing order of their boiling points. [1]
Ans.: i > ii > iii
c) Write the molecular formula of an alcoholic compound having 10 carbon atoms.
Ans.: C10H21OH
Q7. Write the IUPAC name of the following:
a) CH3OH
b) C2H5OH
c) C3H7OH
d) C6H13OH
e) C9H19OH
Ans.:
a) Methanol
b) Ethanol
c) Propanol
d) Hexanol
e) Nonanol
Q8. Write the IUPAC name for the given structure.
a) CH3CH2CH2OH
b) CH3CH=CHCH2CH3
c) CH3CH=CH2
Ans.:
a) Propanol
b) Pent-2-ene/pentene
c) Prop-1- ene / propene
Q9. Represent the condensed formula for
a) Propanol
b) Butanol
Ans.:
a) CH₃CH₂CH₂OH
b) CH₃CH₂CH₂CH2OH
Q10. Write the structural formula for the following compounds.
i) 2-methyl-3-pentanol
ii) Hexan-3-ol
Ans.:
i)

ii)

Compiled from BCSE 2017-2021