
Burning of LPG is the exothermic reaction that releases energy from the chemical bonds present in butane (C4H10) or propane (C3H8).
Energypedia
Multiple Choice Questions
Q1. What does the positive value of ΔH indicate in chemical reaction?
A. release of heat energy
B. absorption of heat energy
C. zero absorption of heat energy
D. zero release of heat energy
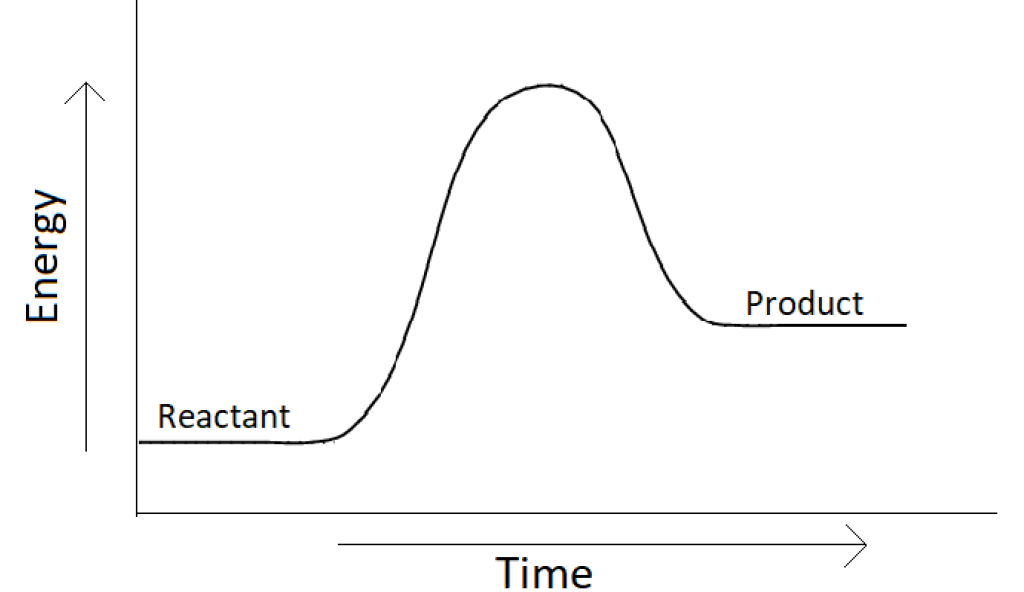
Q2. Which of the following line best describes the energy diagram below?
A. It is an exothermic reaction and the heat is absorbed
B. It is an endothermic reaction and the heat is released
C. It is an exothermic reaction and the heat is released
D. It is an endothermic reaction and the heat is absorbed
Q3. The value of enthalpy in the diagram would be
A. more than zero
B. less than zero
C. equal to zero
D. remain same
Q4. The branch of chemistry that deals with changes in heat energy during the chemical reaction is
A. chemical energetics
B. thermochemistry
C. green chemistry
D. analytical chemistry
Q5. Which of the following is an example of endothermic reaction?
A. Photosynthesis
B. Burning of wood
C. Respiration
D. Fermentation
Q6. Study the given thermochemical equation and identify the type of reaction.
CH4 + 2O2 → 2H2O + CO2 H=728kJmol-1
A. Combustion
B. Dehydration
C. Esterification
D. Oxidation
Q7. Study the given thermochemical equation and identify the type of reaction.
A + B → C + D ΔH= 728kJmol-1
A. Endothermic reaction
B. Irreversible reaction
C. Exothermic reaction
D. Reversible reaction
Q8. All of the following reactions are exothermic in nature; EXCEPT
A. Respiration
B. Burning of magnesium ribbon
C. Formation of calcium hydroxide
D. Decomposition of calcium carbonate.
Q9. An example of an exothermic reaction is
A. melting of ice
B. heating of iodine
C. burning of methane
D. evaporation of water
Q10. The following chemical equation represents a chemical process. What is ‘A’ in the following reaction?
CO2 + 12H2O + ‘A’ → C6H12O6 + 6O2 + 6H2O
A. heat energy
B. light energy
C. electrical energy
D. chemical energy
Q11. A chemistry teacher asked one of the students to dissolve sodium hydroxide pellets in cold water. In due course of stirring the mixture, the student felt the production of heat. What can you conclude from the above experiment?
A. It is due to the heat of neutralization
B. It is due to the endothermic reaction
C. It is due to the exothermic reaction
D. It is due to the stirring of the mixture
Questions
Q1. Fill in the blanks:
a) The energy stored in a substance is __________________.
Ans.: internal energy
b) According to __________________, whenever one form of energy disappears an equivalent amount of some other energy appears.
Ans.: law of conservation of energy
c) The substance that increases the rate of a reaction without undergoing a permanent change is called __________________.
Ans.: catalyst
Q2. Define heat of solution.
Ans.: The amount of heat absorbed or evolved when 1 mole of solute dissolves in the solvent.
Q3. State the law of Conservation of Energy.
Ans.: Energy can neither be created nor destroyed by any physical or chemical change although it can be converted from one form into another.
Q4. When methane is burned with oxygen, it produces carbon dioxide and water vapour. Study the chemical reaction below and answer the questions that follow.
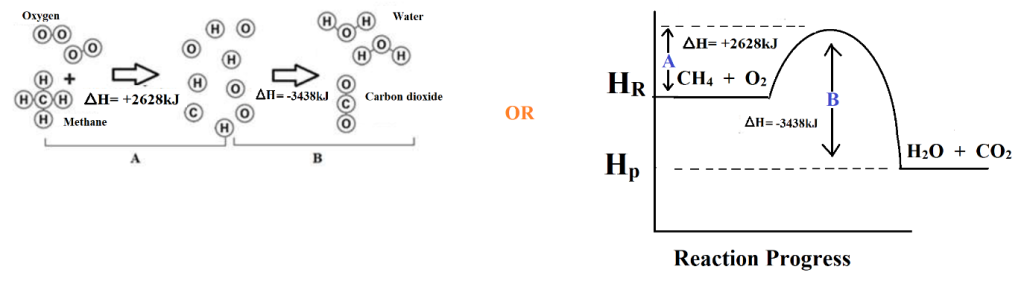
a) Identify the reactions occurring in A and B
Ans.:
A. Endothermic reaction
B. Exothermic reaction
b) Calculate the change in enthalpy for the entire reaction
Ans.: Change in enthalpy is equal to sum of all change in enthalpies (ΣΔH)
ΣΔH= ΔHA + ΔHB
= 2628kJ + (-3438kJ) = –810kJ
Q5. Study the thermochemical equations and answer the following questions.
HCl + NaOH → NaCl + H2O ΔH= -13.7 Kcal
C3H8 + 5O2 → 3CO2 + 4H2O ΔH= -1530.6 Kcal
a) What is the significance of ΔH value in the above reaction?
Ans.: Both the reaction is exothermic or occurs by releasing heat as ΔH value is negative.
b) Classify the above reaction into types of heat of reaction?
Ans.:
Equation (i) is heat of neutralization and
Equation (ii) is heat of combustion.
Q6.When a candle burns in the presence of oxygen, the reaction is exothermic.
Using the above information, answer the following questions:
a) What is the value of ΔH?
Ans.: ΔH = negative.
b) Name the chemical process taking place.
Ans.: Heat of combustion/Exothermic reaction.
Q7. Use the following chemical reaction to answer the questions.
NaCl(s) + (aq) → NaCl(aq) ΔH = +1.2 kcal
HCl(g) + (aq) → HCl(aq) ΔH = -17.51 kcal
a) What does the negative and positive value of ΔH indicate in the above reactions?
Ans.:
Negative value of ΔH = Exothermic reaction
Positive value of ΔH = Endothermic reaction
b) Which one of the above product is stable? Why?
Ans.: HCl product is stable because the ΔH is Negative.
c) Calculate the amount of heat produced when 3 moles of HCl dissolves in water?
Ans.: 3 x 17.51kcal = 52.53 kcal
Q8. Identify the following reactions under exothermic and endothermic:
i) Formation of cations
ii) Formation of anions
iii) Melting of ice
iv) Neutralization of acid by a base.
Ans.:
| Exothermic Reaction | Endothermic Reaction |
| Formation of anions Neutralization of acid by a base. | Formation of cations Melting of ice |
Q9. Wangmo is new comer in your school and she is confused with exothermic and endothermic reaction. How would you help her to differentiate between the two reactions with illustration?
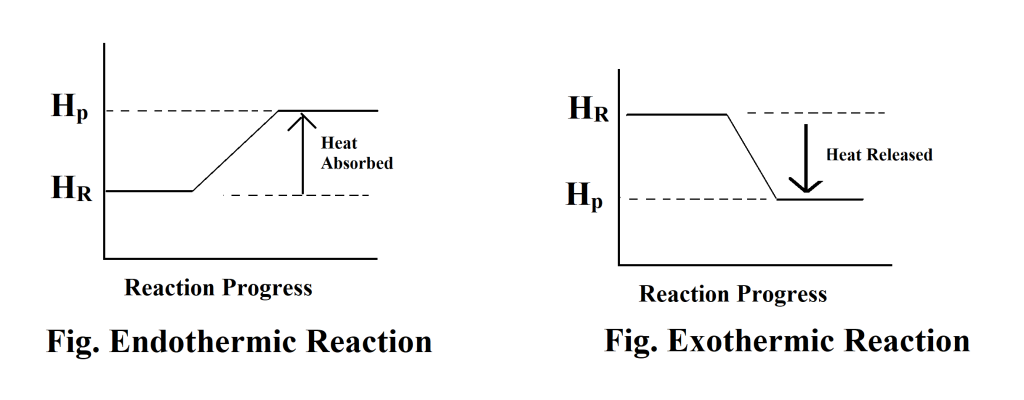
Ans.: Endothermic reaction is reaction in which heat is absorbed.
Exothermic reaction is reaction in which heat is released.
Q10. Explain the energy change during bond breaking and formation of bond.
Ans.: Energy is ABSORBED during the breaking of bond and energy is RELEASED while the formation of bond.
Bond breaking is endothermic reaction (ΔH = Positive) while formation of bond is exothermic reaction (ΔH = Negative).
Compiled from BCSE 2017-2021